-
 Scholz urges Ukraine talks in first call with Putin since 2022
Scholz urges Ukraine talks in first call with Putin since 2022
-
Zverev reaches ATP Finals last four, Alcaraz on brink of exit

-
 Lebanon rescuer picks up 'pieces' of father after Israel strike
Lebanon rescuer picks up 'pieces' of father after Israel strike
-
US retail sales lose steam in October after hurricanes

-
 Zverev reaches ATP Finals last four with set win against Alcaraz
Zverev reaches ATP Finals last four with set win against Alcaraz
-
Kerevi back for Australia against Wales, Suaalii on bench

-
 Spate of child poisoning deaths sparks S.Africa xenophobia
Spate of child poisoning deaths sparks S.Africa xenophobia
-
Comedian Conan O'Brien to host Oscars

-
 Rozner overtakes McIlroy and Hatton for Dubai lead
Rozner overtakes McIlroy and Hatton for Dubai lead
-
Mourners bid farewell to medic killed in east Ukraine

-
 Gore says 'absurd' to hold UN climate talks in petrostates
Gore says 'absurd' to hold UN climate talks in petrostates
-
Hamas says 'ready for ceasefire' as Israel presses Gaza campaign

-
 Amorim says Man Utd is 'where I'm supposed to be'
Amorim says Man Utd is 'where I'm supposed to be'
-
Japan hammer Indonesia to edge closer to World Cup spot

-
 Jeff Beck guitar collection to go under the hammer in January
Jeff Beck guitar collection to go under the hammer in January
-
Veteran Ranieri has 'no time for mistakes' on Roma return

-
 Van Nistelrooy says he will 'cherish' Man Utd memories in farewell message
Van Nistelrooy says he will 'cherish' Man Utd memories in farewell message
-
IAEA chief tours sensitive Iran nuclear plants

-
 Pompeii rejects 'mass tourism' with daily visitor limit
Pompeii rejects 'mass tourism' with daily visitor limit
-
Jailed Russian poet could be 'killed' in prison, warns wife

-
 French court orders release of Lebanese militant held since 1984
French court orders release of Lebanese militant held since 1984
-
Global stocks struggle after Fed signals slower rate cuts

-
 UK economy slows, hitting government growth plans
UK economy slows, hitting government growth plans
-
Primary schools empty as smog persists in Indian capital

-
 Palestinians turn to local soda in boycott of Israel-linked goods
Palestinians turn to local soda in boycott of Israel-linked goods
-
Typhoon Man-yi bears down on Philippines still reeling from Usagi

-
 UK growth slows in third quarter, dealing blow to Labour government
UK growth slows in third quarter, dealing blow to Labour government
-
Chris Wood hits quickfire double in NZ World Cup qualifying romp

-
 Markets struggle at end of tough week
Markets struggle at end of tough week
-
China tests building Moon base with lunar soil bricks

-
 Film's 'search for Palestine' takes centre stage at Cairo festival
Film's 'search for Palestine' takes centre stage at Cairo festival
-
Oil execs work COP29 as NGOs slam lobbyist presence

-
 Gore says climate progress 'won't slow much' because of Trump
Gore says climate progress 'won't slow much' because of Trump
-
'Megaquake' warning hits Japan's growth

-
 Stiff business: Berlin startup will freeze your corpse for monthly fee
Stiff business: Berlin startup will freeze your corpse for monthly fee
-
Wars, looming Trump reign set to dominate G20 summit

-
 Xi, Biden attend Asia-Pacific summit, prepare to meet
Xi, Biden attend Asia-Pacific summit, prepare to meet
-
Kyrgios to make competitive return at Brisbane next month after injuries

-
 Dominican Juan Luis Guerra triumphs at 25th annual Latin Grammys
Dominican Juan Luis Guerra triumphs at 25th annual Latin Grammys
-
Landslide win for Sri Lanka president's leftist coalition in snap polls

-
 Australian World Cup penalty hero Vine takes mental health break
Australian World Cup penalty hero Vine takes mental health break
-
As Philippines picks up from Usagi, a fresh storm bears down

-
 Tropical Storm Sara pounds Honduras with heavy rain
Tropical Storm Sara pounds Honduras with heavy rain
-
Pepi gives Pochettino win for USA in Jamaica

-
 'Hell to heaven' as China reignite World Cup hopes with late winner
'Hell to heaven' as China reignite World Cup hopes with late winner
-
Rebel attacks keep Indian-run Kashmir on the boil

-
 New Zealand challenge 'immense but fantastic' for France
New Zealand challenge 'immense but fantastic' for France
-
Under pressure England boss Borthwick in Springboks' spotlight

-
 All Blacks plan to nullify 'freakish' Dupont, says Lienert-Brown
All Blacks plan to nullify 'freakish' Dupont, says Lienert-Brown
-
TikTok makes AI driven ad tool available globally

| RIO | 1.08% | 61.09 | $ | |
| CMSC | 0.04% | 24.56 | $ | |
| NGG | 0.22% | 62.509 | $ | |
| SCS | 0.38% | 13.32 | $ | |
| CMSD | 0.39% | 24.4525 | $ | |
| GSK | -2.74% | 33.095 | $ | |
| BP | 0.27% | 29.13 | $ | |
| BTI | 1.87% | 36.165 | $ | |
| RBGPF | 2.67% | 61.84 | $ | |
| RYCEF | 0.88% | 6.85 | $ | |
| BCC | -0.21% | 140.05 | $ | |
| JRI | -0.2% | 13.05 | $ | |
| AZN | -2.14% | 63.68 | $ | |
| BCE | -0.92% | 26.595 | $ | |
| VOD | 1.08% | 8.775 | $ | |
| RELX | -3.42% | 44.43 | $ |

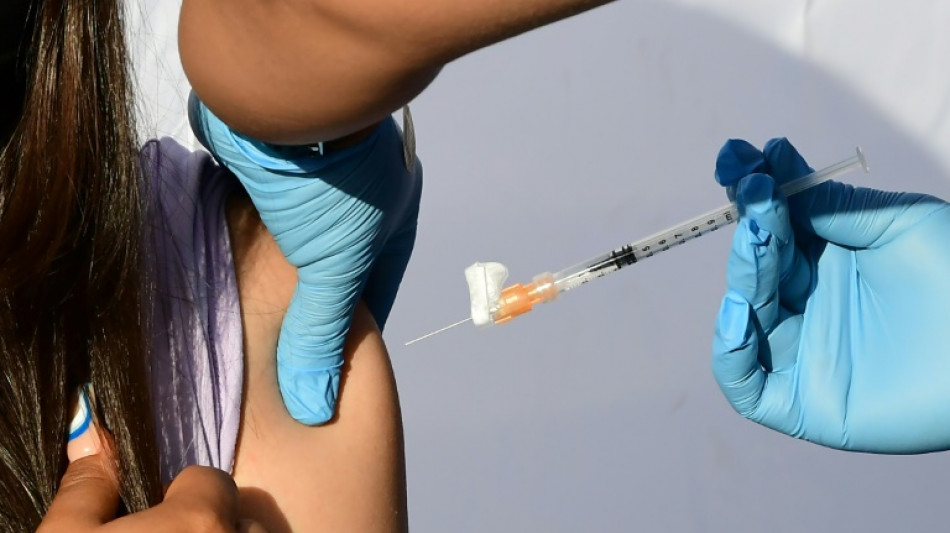
US FDA says Pfizer Covid vaccine effective in kids under five
The US Food and Drug Administration (FDA) has said the Pfizer Covid vaccine is safe and effective in children under five, ahead of a meeting to weigh its authorization later this week.
Children under five are the only age group not yet eligible for vaccination in the United States and most countries, a pressing need since rates of hospitalization and death "are higher than among children and adolescents 5-17 years of age," the FDA said in a document posted on its website Sunday.
The agency has called a meeting of experts on June 15 to decide whether to recommend the Pfizer vaccine, given as three shots to children aged six months through four years, as well as the Moderna vaccine, given as two shots to children aged six months through five years.
Pfizer's first two shots are given three weeks apart, then the third is given eight weeks after the second. They are all dosed at three micrograms, as opposed to 30 micrograms the company gives older ages.
Both Pfizer and Moderna had previously posted their results, but the FDA then had to review the data in detail and carry out its own evaluation. It posted a favorable analysis about Moderna on Friday.
Its comments towards Pfizer also appear favorable, based on the levels of infection-blocking antibodies it evoked in trial participants, and a similar side-effect profile to higher age groups. The trial population was around 4,500 children.
A preliminary estimate placed vaccine efficacy at 80.3 percent, but the FDA noted this was based on very few positive cases -- just 10, as opposed to the 21 sought for a more accurate figure.
There are nearly 20 million US children aged four years and under, or six percent of the population. If, as expected, the FDA-appointed experts recommend the two shots, then the matter will go to another committee convened by the Centers for Disease Control and Prevention for a final say.
White House officials last week said rollout of millions of shots at pharmacies and doctors' offices could begin as soon as June 21, following the Juneteenth holiday on June 20.
M.King--AT




