-
 Dutch coalition survives political turmoil after minister's resignation
Dutch coalition survives political turmoil after minister's resignation
-
Uruguay end winless run with dramatic late win over Colombia

-
 Max potential: 10 years since a teenage Verstappen wowed in Macau
Max potential: 10 years since a teenage Verstappen wowed in Macau
-
Tens of thousands flee as Typhoon Man-yi nears Philippines

-
 Is Argentina's Milei on brink of leaving Paris climate accord?
Is Argentina's Milei on brink of leaving Paris climate accord?
-
Big Bang: Trump and Musk could redefine US space strategy

-
 Revolution over but more protests than ever in Bangladesh
Revolution over but more protests than ever in Bangladesh
-
Minister resigns but Dutch coalition remains in place

-
 Ireland won 'ugly', says relieved Farrell
Ireland won 'ugly', says relieved Farrell
-
Stirring 'haka' dance disrupts New Zealand's parliament

-
 England's Hull grabs lead over No.1 Korda at LPGA Annika
England's Hull grabs lead over No.1 Korda at LPGA Annika
-
Kosovo players walk off in Romania after 'Serbia' chants, game abandoned

-
 Kosovo players walk off in Romania game after 'Serbia' chants
Kosovo players walk off in Romania game after 'Serbia' chants
-
Lame-duck Biden tries to reassure allies as Trump looms

-
 Nervy Irish edge Argentina in Test nailbiter
Nervy Irish edge Argentina in Test nailbiter
-
Ronaldo at double as Portugal reach Nations League quarters, Spain win

-
 Fitch upgrades Argentina debt rating amid economic pain
Fitch upgrades Argentina debt rating amid economic pain
-
Trump picks Doug Burgum as energy czar in new administration

-
 Phone documentary details struggles of Afghan women under Taliban
Phone documentary details struggles of Afghan women under Taliban
-
Ronaldo shines as Portugal rout Poland to reach Nations League last-eight

-
 Spain beat Denmark to seal Nations League group win
Spain beat Denmark to seal Nations League group win
-
Former AFCON champions Ghana bow out as minnows Comoros qualify

-
 Poland, Britain reach BJK Cup quarter-finals
Poland, Britain reach BJK Cup quarter-finals
-
At summit under Trump shadow, Xi and Biden signal turbulence ahead

-
 Lebanon said studying US truce plan for Israel-Hezbollah war
Lebanon said studying US truce plan for Israel-Hezbollah war
-
Xi warns against 'protectionism' at APEC summit under Trump cloud

-
 Nigerian UN nurse escapes jihadist kidnappers after six years
Nigerian UN nurse escapes jihadist kidnappers after six years
-
India in record six-hitting spree to rout South Africa

-
 George tells England to prepare for rugby 'war' against Springboks
George tells England to prepare for rugby 'war' against Springboks
-
Pogba's Juve contract terminated despite doping ban reduction

-
 Ukraine slams Scholz after first call with Putin in two years
Ukraine slams Scholz after first call with Putin in two years
-
Michael Johnson's Grand Slam Track series to have LA final

-
 Kagiyama, Yoshida put Japan on top at Finland Grand Prix
Kagiyama, Yoshida put Japan on top at Finland Grand Prix
-
Alcaraz eyeing triumphant Davis Cup farewell for Nadal after ATP Finals exit

-
 Xi, Biden at Asia-Pacific summit under Trump trade war cloud
Xi, Biden at Asia-Pacific summit under Trump trade war cloud
-
India go on record six-hitting spree against South Africa

-
 France skipper Dupont says All Blacks 'back to their best'
France skipper Dupont says All Blacks 'back to their best'
-
Trump pressures US Senate with divisive cabinet picks

-
 Bagnaia strikes late in Barcelona practice to edge title rival Martin
Bagnaia strikes late in Barcelona practice to edge title rival Martin
-
High-ball hero Steward ready to 'front up' against South Africa

-
 Leader of Spain flood region admits 'mistakes'
Leader of Spain flood region admits 'mistakes'
-
Swiatek, Linette take Poland past Spain into BJK Cup quarter-finals

-
 Leftist voices seek to be heard at Rio's G20 summit
Leftist voices seek to be heard at Rio's G20 summit
-
Wales coach Jenkins urges players to 'get back on the horse'

-
 Zverev reaches ATP Finals last four, Alcaraz out
Zverev reaches ATP Finals last four, Alcaraz out
-
Boeing strike will hurt Ethiopian Airlines growth: CEO

-
 Springboks skipper Kolisi wary of England's 'gifted' Smith
Springboks skipper Kolisi wary of England's 'gifted' Smith
-
End of a love affair: news media quit X over 'disinformation'

-
 US finalizes up to $6.6 bn funding for chip giant TSMC
US finalizes up to $6.6 bn funding for chip giant TSMC
-
Scholz urges Ukraine talks in first call with Putin since 2022


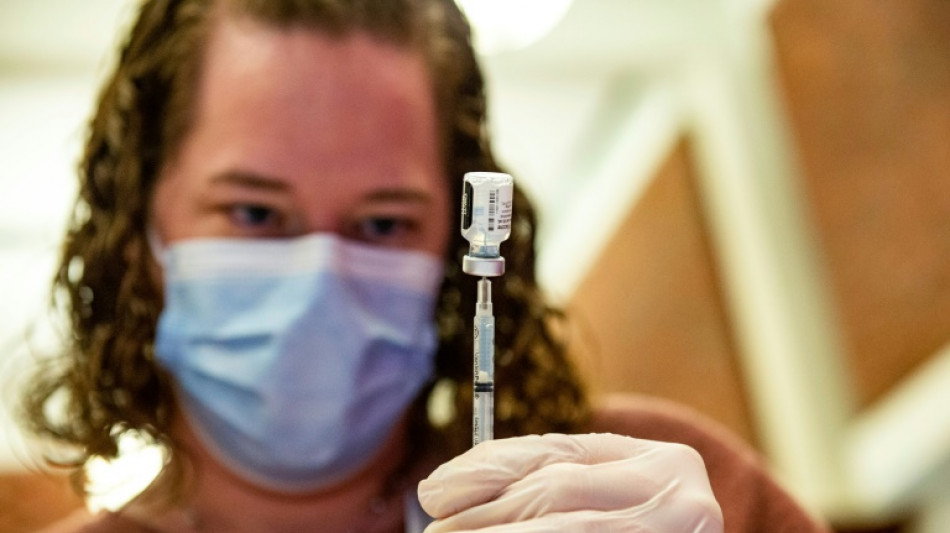
US approves shots targeting Omicron
US officials Wednesday authorized updated Covid-19 vaccinations by Moderna and Pfizer-BioNTech that specifically target the latest strains of the Omicron variant, hoping to contain a new wave of feared contagions this winter.
The two new booster shots are approved for people age 12 and above for the Pfizer shot and 18 and older for Moderna, the Food and Drug Administration (FDA) said in a statement.
This new generation of so-called "bivalent" vaccines protects against both the original strain of Covid and the BA.4 and BA.5 lineages -- the subvariants of Omicron which account for about 90 percent of all new cases in the United States.
"Although the current Covid-19 surge is waning overall, it's predicated that we'll enter yet another surge as we spend more time indoors later this fall and winter," FDA Commissioner Robert Califf told reporters.
"These update boosters present us with an opportunity to get ahead" of the curve, he said.
While the intense focus on coronavirus has largely faded from daily life for Americans, the United States still records some 80,000 new cases -- and 400 deaths -- from Covid every day.
Earlier this summer the US health department announced it had purchased 105 million doses from Pfizer and 66 million from Moderna for use over the fall and winter.
The vaccines must still receive a recommendation by the Centers for Disease Control and Prevention, the nation's health protection agency, before injections can begin.
An independent panel of experts is scheduled to be convened by the CDC on Thursday to discuss the updates.
- Low booster uptake -
The two companies indicated their updated vaccines could be available for distribution in the United States as early as next week.
"Receiving a booster that specifically targets the Omicron BA.4/.5 variant... is an important public health measure that people can take to help protect themselves," Moderna chief executive Stephane Bancel said in a statement.
Many Americans will need convincing to take the new shots, as how only about half of those eligible have received a first booster dose.
The vaccines currently in circulation target the initial strain of the virus that first appeared in Wuhan, China. They have gradually proven to be less effective against the variants that have appeared over time, due to rapid evolution of the virus.
The FDA still recommends people get the original vaccine in order to receive "a foundation of that basic immune response," Califf said.
In contrast to the Alpha and Delta variants, which eventually waned, Omicron and its subvariants have come to dominate infections worldwide in 2022.
Pfizer and Moderna have also filed for approval of their updated vaccines with the European Medicines Agency.
R.Chavez--AT




